神経誘導は、デフォルトが神経系への分化でありそれをBMPシグナルが表皮になるようにしているところ、BMP阻害因子であるNoggin, Chordin, Follistatinなど作用することでBMPシグナルを抑えた領域が神経系になるという「神経分化デフォルト」説で一般的に説明されます。しかしFGFが神経誘導に関与するという報告もあり、必ずしも統一的な見方が確立しているように思えません。
神経誘導因子としてのnogginの局在
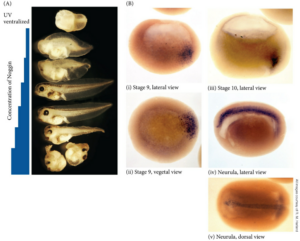 Gilbert Developmental Biology FIGURE 12.21 noggin mRNAインジェクションによるUV腹側化胚の背側構造レスキュー(A)、内在性noggin mRNAの局在(B)。(B) Localization of noggin mRNA in the organizer tissue, shown by in situ hybridization. At gastrulation (i and ii, stage 9), noggin mRNA (dark areas) accumulates in the dorsal marginal zone. When cells involute (i and iii, stages 9 and 10), noggin mRNA is seen in the dorsal blastopore lip. During convergent extension (iii, stage 10), noggin is expressed in the precursors of the notochord, prechordal plate, and pharyngeal endoderm, which in the neurula (iv, v) extend beneath the ectoderm in the center of the embryo. Barresi, Michael; Gilbert, Scott. Developmental Biology XE (English Edition) (p.3663). Sinauer Associates is an imprint of Oxford University Press. Kindle 版.
Gilbert Developmental Biology FIGURE 12.21 noggin mRNAインジェクションによるUV腹側化胚の背側構造レスキュー(A)、内在性noggin mRNAの局在(B)。(B) Localization of noggin mRNA in the organizer tissue, shown by in situ hybridization. At gastrulation (i and ii, stage 9), noggin mRNA (dark areas) accumulates in the dorsal marginal zone. When cells involute (i and iii, stages 9 and 10), noggin mRNA is seen in the dorsal blastopore lip. During convergent extension (iii, stage 10), noggin is expressed in the precursors of the notochord, prechordal plate, and pharyngeal endoderm, which in the neurula (iv, v) extend beneath the ectoderm in the center of the embryo. Barresi, Michael; Gilbert, Scott. Developmental Biology XE (English Edition) (p.3663). Sinauer Associates is an imprint of Oxford University Press. Kindle 版.
上のデータを見ると、nogginは見事に脊索前板、脊索など神経板の裏側で発現しており、神経誘導シグナルと言われると納得できます。
Nodalnの2つの役割:中胚葉誘導と左右の形成
Nodalは左右の非対称性の形成で重要な役割を担いますが、背側中胚葉の誘導においても大事な役割があります。2つの全然異なる現象に関与しているので、nodalに関する文献を漫然と見ていると頭がこんがらがってきます。
TGFß signals belonging to the Nodal family set up the embryonic axes, induce mesoderm and endoderm, pattern the nervous system, and determine left-right asymmetry in vertebrates.
Annual Review of Cell and Developmental Biology Volume 19, 2003 Review Article Nodal Signaling in Vertebrate Development Alexander F. Schierhttps://www.annualreviews.org/content/journals/10.1146/annurev.cellbio.19.041603.094522 本文有料
Nodal-related 1 (ndr1) and nodal-related 2 (ndr2)genes in zebrafish encode members of the nodal subgroup of the transforming growth factor-β superfamily. We report the expression patterns and functional characteristics of these factors, implicating them in the establishment of dorsal–ventral polarity and left–right asymmetry.
Zebrafish Nodal-Related Genes Are Implicated in Axial Patterning and Establishing Left–Right Asymmetry Michael R. Rebagliati a , Reiko Toyama a , Cornelia Fricke b , Pascal Haffter b , Igor B. Developmental Biology Volume 199, Issue 2, 15 July 1998, Pages 261-272 Dawid https://www.sciencedirect.com/science/article/pii/S0012160698989357?via%3Dihub
左右軸形成における役割
下の論文はよくまとまったレビューだと思います。原始結節で生み出された左右差がどのようにして側板中胚葉Lateral Plate Mesodermにつたわるのかは謎だとしています。
Follow your gut: Relaying information from the site of left–right symmetry breaking in the mouse Yukio Saijoh, Manuel Viotti, Anna-Katerina Hadjantonakis First published: 19 April 2014 https://doi.org/10.1002/dvg.22783
Snail family genes are required for left–right asymmetry determination, but not neural crest formation, in mice Stephen A. Murray and Thomas Gridley tom.gridley@jax.orgAuthors Info & Affiliations Edited by Kathryn V. Anderson, Sloan–Kettering Institute, New York, NY, and approved May 26, 2006 July 5, 2006 103 (27) 10300-10304 PNAS https://www.pnas.org/doi/10.1073/pnas.0602234103
Nodalの神経誘導作用
下の論文の図のd Control k81(表皮のマーカー)、sox2(神経板のマーカー)を見ると、外胚葉が表皮と神経板とに分化した様子が見事にわかります。非常に興味深いのはNodalを胞胚腔に注入した胚における神経板の広がりです。表皮の部分が狭まり、その分、神経板が大きく広がっています。つまり、Nodalは強力な神経誘導作用を持つと言えます。ただし、直接的な作用なのか、間接的な作用(誘導因子を誘導して)なのかの区別がメカニズムを考えるうえで重要になります。タンパク質を胞胚腔に顕微注入しているので、内胚葉、中胚葉、外胚葉全てに作用しうる実験条件だと思います。
Nodal/Activin Pathway is a Conserved Neural Induction Signal in Chordates Nat Ecol Evol. 2017 Jul 3;1(8):1192–1200. doi: 10.1038/s41559-017-0226-3 図の説明: d, Expression of sox2 and k81 in control embryos and in embryos injected with zBMP4, nodal or both recombinant proteins. Scale bar, 250 μm. 方法: Xenopus embryos were injected in the blastocoel with 10 ng mouse recombinant Nodal protein (R&D), 3.5 ng zebrafish recombinant BMP4 protein (R&D), or 30 ng recombinant human Noggin (R&D).
アフリカツメガエルにはNodalは複数種類発現しており、Nodal-related番号 で呼ばれています。
Fig. 4. Temporal and spatial expression of Xnr-1 and Xnr-2 during Xenopus development.(B-H) Whole-mount in situ hybridization analysis of Xnr-1 and Xnr-2 expression. All embryos are cleared albino embryos, viewed from the vegetal surface with dorsal oriented upward. The dorsal lip is indicated by the black arrowhead. (B) Stage 9 embryos show punctate perinuclear Xnr-1 signal over the entire vegetal region. Xnr-2 shows the same pattern (data not shown). (C) Xnr-1 signal at stage 10.25 is restricted to the dorsal marginal zone (dark arc at bottom left is a background artefact). (D) Xnr-2 signal in stage 10 pregastrula is primarily located in the dorsal marginal zone, but also in adjacent dorsovegetal cells. (E) Xnr-2 signal in the stage 10.5 gastrula is highly concentrated just above the dorsal lip, with a gradual decrease laterally and ventrally. (F) Whole-mount stained stage 10.25 embryo, split open along the dorsal/ventral plane and viewed internally to show Xnr-2 expression at the dorsal lip. Superficial and slightly deeper staining is observed. Some out-of-focus vegetal cells below the lip express Xnr-2 (white arrowhead). https://journals.biologists.com/dev/article/121/11/3651/38534/Nodal-related-signals-induce-axial-mesoderm-and
Nodalは名前が示すようにマウスのnode(原始結節)に発現する遺伝子です。Nodalは、外胚葉に直接働きかけて神経を誘導するわけではありませんん。もっと前の段階すなわち、いわゆるオーガナイザーを誘導する因子という位置付けだと思います。
- Several TGFβ ligands present in the blastula embryo, including Activin, Vg1, Derriere and the Nodal-related factors Xnr1 and Xnr2, each have the ability to induce the expression of both general and organizer-specific mesodermal markers (Asashima et al., 1990; Smith et al., 1990; Thomsen et al., 1990; Thomsen and Melton, 1993; Jones et al., 1995; Kessler and Melton, 1995; Sun et al., 1999).
- Genetic studies in the mouse and zebrafish demonstrate a requirement for Nodal-related genes in mesoderm and organizer formation (Schier and Shen, 2000).
- Loss-of-function mutations in the mouse and the zebrafish Nodal genes result in embryos which fail to form an organizer and lack mesoderm (Conlon et al., 1994; Feldman et al., 1998). Likewise, inhibition of Nodal signaling in Xenopus, using a Nodal-specific form of Cerberus, blocks mesoderm and organizer formation (Agius et al., 2000).
- While Nodal signaling induces mesoderm in the equatorial region of the blastula, the organizer forms in a dorsal equatorial domain in response to maternal Wnt signaling (reviewed in Harland and Gerhart, 1997; Heasman, 1997; Moon and Kimelman, 1998).
- Wnt3 and Nodal function are required in the mouse for gastrulation and node formation (Liu et al., 1999; Conlon et al., 1994),
Cooperation of Siamois and TGFβ signals Siamois cooperates with TGFβ signals to induce the complete function of the Spemann-Mangold Organizer MARK J. ENGLEKA and DANIEL S. KESSLE Int. J. Dev. Biol. 45: 241-250 (2001) 241
アフリカツメガエルの場合Nodalは複数発現していますが、Xnr3はオーガナイザー領域に発現しているようです(下の論文の図B,C)
Fig. 2. The spatial distribution of Xnr5 and Xnr6 expression was analyzed by whole-mount in situ hybridization as previously described (Harland, 1991). The spatial distribution of Xnr5 and Xnr6 expression was analyzed by whole-mount in situ hybridization as previously described (Harland, 1991). In the late blastula embryo, Xnr5 and Xnr6 mRNA was detected from the vegetal pole to the dorsal vegetal region, including the Nieuwkoop center (Fig. 2D-F). At early gastrula, an Xnr6 signal was seen at just beneath the dorsal lip (Fig. 2G), whereas no Xnr5 signal could be detected at this stage (data not shown). Unlike Xnr3 (Fig. 2B,C), Xnr5 and Xnr6 transcripts did not localize at the Spemann’s organizer.
Secondary axis induction by Xnr5 and Xnr6. https://journals.biologists.com/dev/article/127/24/5319/41043/Two-novel-nodal-related-genes-initiate-early
These results indicate that the structures of Xnr5 and Xnr6 are quite similar to Xnr1 and Xnr2. All of these four factors can induce a secondary axis lacking the head structures when they are misexpressed ventrally. Xnr4 also has similar activity (S. T., C. Y., Y. O. and M. A., unpublished).
https://journals.biologists.com/dev/article/127/24/5319/41043/Two-novel-nodal-related-genes-initiate-early
ノーダル阻害剤:Cer-S
Cerberus is a head-inducing secreted factor (Bouwmeester et al., 1996) that acts as a multifunctional antagonist of Nodal, BMP (Bone Morphogenetic Proteins) and Wnt signals (Hsu et al., 1998; Piccolo et al., 1999). A carboxy-terminal fragment of Cerberus, called Cerberus-short (Cer-S), lacks the anti-Wnt and anti-BMP activities but retains full anti-Xnr1 activity (Piccolo et al., 1999). In biochemical studies, Cer-S was found to bind Xnr1, but not Activin nor Vg1 proteins (Piccolo et al., 1999). https://pmc.ncbi.nlm.nih.gov/articles/PMC2292107/
この論文(↓)ではNordalがニュークープセンターの実体を担う分子として中胚葉誘導(背側、すなわちオーガナイザー)を起こすか、そもそも内胚葉背側に存在するかを調べています。
Endodermal Nodal-related signals and mesoderm induction in Xenopus Eric Agius 1,*,‡, Michael Oelgeschläger 1,*, Oliver Wessely 1, Caroline Kemp 1, E M De Robertis 1,§ Development. 2000 Mar;127(6):1173–1183. doi: 10.1242/dev.127.6.1173
- Smad2 mediates Activin/Nodal signaling in mesendoderm differentiation of mouse embryonic stem cells Teng Fei, Shanshan Zhu, Kai Xia, Jianping Zhang, Zhongwei Li, Jing-Dong J Han & Ye-Guang Chen Cell Research volume 20, pages1306–1318 (2010) https://www.nature.com/articles/cr2010158
- FELDMAN, B., GATES, M.A., EGAN, E.S., DOUGAN, S.T., RENNEBECK, G., SIROTKIN, H.I., SCHIER, A.F. and TALBOT, W.S. (1998). Zebrafish organizer development and germ-layer formation require nodal-related signals. Nature 395: 181-185.




