ヒトの発生は、受精後3週目に中胚葉誘導、原腸陥入が起こります。ヒトで原腸陥入の時期の観察をすることは不可能なので、同時期の動物モデルからヒトの場合を類推するしかありません。
原始線条 primitive streak
ニワトリ卵の原始線条 primitive streak形成の様子。
Chick Primitive Streak BioProfessor101 チャンネル登録者数 2.15万人
- Migrating mesoderm cells self-organize into a dynamic meshwork structure during chick gastrulation Yukiko Nakaya, Mitsusuke Tarama, Sohei Tasaki, Ayako Isomura, View ORCID ProfileTatsuo Shibata doi: https://doi.org/10.1101/2022.09.08.507227 Posted September 09, 2022. https://www.biorxiv.org/content/10.1101/2022.09.08.507227v1.full
- Cell movement during chick primitive streak formation Developmental Biology Volume 296, Issue 1, 1 August 2006, Pages 137-149 細胞の動きを示したsupplementary movie 動画がいくつかあります https://www.sciencedirect.com/science/article/pii/S0012160606007299
- Cell Movement Patterns during Gastrulation in the Chick Are Controlled by Positive and Negative Chemotaxis Mediated by FGF4 and FGF8 Developmental Cell Volume 3, Issue 3, September 2002, Pages 425-437 https://www.sciencedirect.com/science/article/pii/S1534580702002563 細胞移動の軌跡を示した動画がいくつかあります。FGF8とFGF4の役割についても非常にわかりやすい実験結果
カエルの発生を高校で学びますが、両生類の場合は原口というものがあらわれて、外胚葉の細胞がそこから内部に潜り込んでいって中胚葉の細胞に変化するのでした。しかし鳥類や哺乳類は、原口に相当するものが、原始線条(原条)になります。形が違いますが、細胞が潜り込んでいく場所という点で対応する構造だと考えられます。カエルの原口に馴染んでいた身としてはそれに相当するものが原始線条だといわれてもどうもピンときませんでした。馴染むためには繰り返し勉強するしかありません。
ヒトに近い動物モデルとしてはマウスがありますが、ヒトの胚盤が「円盤」なのに対して、マウスは「カップ状」という形態的に大きな違いがあります。下の図を見ると、proamniotic cavity(羊膜腔になる予定の空間)が確かにカップ状のepiblastによって囲まれているのがわかります。下のCell論文の図は非常に明解に描かれています。basal membrane と apical 側との違いもわかりやすいと思います。カップ状になる過程が描かれています。
Self-Organizing Properties of Mouse Pluripotent Cells Initiate Morphogenesis upon Implantation Cell Volume 156, Issue 5p1032-1044February 27, 2014 https://www.cell.com/fulltext/S0092-8674%2814%2900075-0
AEV特異的マーカータンパク質の早期の時期における発現
- AVE protein expression and visceral endoderm cell behavior during anterior-posterior axis formation in mouse embryos: Asymmetry in OTX2 and DKK1 expression. Developmental biology Hideharu Hoshino et al. 36 citations 2015 https://www.sciencedirect.com/science/article/pii/S0012160615001748 AVEマーカータンパク質のステージE4.5の時期の発現

遠位内臓内胚葉(Distal Visceral Endoderm, DVE)
マウス胚における遠位内臓内胚葉(Distal Visceral Endoderm, DVE)は、胚の前後軸(Anterior-Posterior, A-P)形成において重要な役割を果たします。DVEは、特定の遺伝子を発現し、将来の前部内臓内胚葉(Anterior Visceral Endoderm, AVE)へと移動します。このプロセスに関与する遺伝子の発現パターンを理解することは、胚の発生メカニズムを解明する上で重要です。
OTX2, HHEX, CER1, LEFTY1, DKK1の発現:
DVEは、OTX2, HHEX, CER1, LEFTY1, DKK1を発現します。これらの遺伝子は、E4.5からE6.5の間に特定のパターンで発現し、AVEの形成に寄与します。
- AVE protein expression and visceral endoderm cell behavior during anterior-posterior axis formation in mouse embryos: Asymmetry in OTX2 and DKK1 expression. Developmental biology Hideharu Hoshino et al. 36 citations 2015 https://www.sciencedirect.com/science/article/pii/S0012160615001748 ステージE5.25-5.75でのAVEタンパク質のDVEにおける発現
 OTX2、HHEX、CER1、LEFTY1、および DKK1 は、マウス胚の遠位内臓内胚葉 (DVE) で発現します。
OTX2、HHEX、CER1、LEFTY1、および DKK1 は、マウス胚の遠位内臓内胚葉 (DVE) で発現します。
LEFTY1の非対称発現:
LEFTY1は、DVEおよびAVEのマーカーとして機能し、E3.5からE5.5の間に非対称に発現します。この非対称性は、Nodalシグナルによって誘導され、DVEの移動方向を決定します。
- The Mouse Embryo Autonomously Acquires Anterior-Posterior Polarity at Implantation Developmental Cell Volume 10, Issue 4P451-459April 2006 https://www.cell.com/action/showPdf?pii=S1534-5807%2806%2900114-6
Nodalシグナルの役割:
Nodalシグナルは、DVEの生成と移動に重要な役割を果たします。Nodal遺伝子は、内胚葉および多能性エピブラストで発現し、DVE/AVEの移動を駆動します。
-
- Nodal signaling from the visceral endoderm is required to maintain Nodal gene expression in the epiblast and drive DVE/AVE migration Developmental Biology Volume 400, Issue 1, 1 April 2015, Pages 1-9 Developmental Biology https://pdf.sciencedirectassets.com/272543/1-s2.0-S0012160615X00054/1-s2.0-S0012160614006460/main.pdf
Hex遺伝子の発現:
Hexは、E5.5でDVEの一部として発現し、その後、前部に移動します。Hexの発現は、胚の前後軸の最も早期の分子非対称性を示します。
- Hex: a homeobox gene revealing peri-implantation asymmetry in the mouse embryo and an early transient marker of endothelial cell precursors Development . 1998 Jan;125(1):85-94. doi: 10.1242/dev.125.1.85. https://journals.biologists.com/dev/article/125/1/85/39778/Hex-a-homeobox-gene-revealing-peri-implantation
新規遺伝子の発見:
Adtk1などの新規遺伝子がAVEで発現し、これらの遺伝子は胚の発生において重要な役割を果たす可能性があります。
マウス胚の遠位内臓内胚葉(DVE)は、OTX2, HHEX, CER1, LEFTY1, DKK1などの遺伝子を発現し、これらの遺伝子はDVEの移動と前後軸の形成に重要な役割を果たします。特にLEFTY1とNodalシグナルは、DVEの非対称性と移動方向を決定する上で重要です。さらに、HexやAdtk1などの遺伝子もDVEおよびAVEの形成に関与しています。(consensus.ai)
DVEの移動:AVE
Distal Vesceral Endoderm (DVE)の細胞が最初はカップ状の胚の極のところに生じますが、それが端に移動してAnterior Viscereal Endoderm (AVE)になります。
From fertilization to gastrulation: axis formation in the mouse embryo. Curr Opin Genet Dev . 2001 Aug;11(4):384-92. DOI:10.1016/S0959-437X(00)00208-2Corpus ID: 17925624
Vertebrate Axial Patterning: From Egg to Asymmetry. Advances in Experimental Medicine and Biology, 01 Jan 2017, 953:209-306 https://doi.org/10.1007/978-3-319-46095-6_6 PMID: 27975274 PMCID: PMC6550305 https://europepmc.org/article/pmc/pmc6550305
DVEがAVEの位置に移動するためにはnodalシグナルが必要なようです。
- Nodal signaling from the visceral endoderm is required to maintain Nodal gene expression in the epiblast and drive DVE/AVE migration Developmental Biology Volume 400, Issue 1, 1 April 2015, Pages 1-9 Developmental Biology https://pdf.sciencedirectassets.com/272543/1-s2.0-S0012160615X00054/1-s2.0-S0012160614006460/main.pdf
AVEで発現する遺伝子
- AVE protein expression and visceral endoderm cell behavior during anterior-posterior axis formation in mouse embryos: Asymmetry in OTX2 and DKK1 expression. Developmental biology Hideharu Hoshino et al. 36 citations 2015 https://www.sciencedirect.com/science/article/pii/S0012160615001748 ステージE6.5でのAVEにおける特異的なタンパク質の発現

AVEが発現する分泌シグナル分子
マウスの初期胚における前部内臓内胚葉(AVE)は、胚の前後軸の形成に重要な役割を果たします。AVEは、成長因子シグナルを拮抗する分泌因子を発現することでその機能を果たします。
- Wntシグナルの拮抗因子:Secreted frizzled-related protein 5 (Sfrp5) は、Wntシグナルを拮抗する分泌因子であり、AVEおよび前腸内胚葉で発現します。Dkk1もまた、Wntシグナルを拮抗する分泌因子であり、AVEで発現します。
- TGFβシグナルの拮抗因子:AVEは、Nodalシグナルを抑制するTGFβ拮抗因子を分泌します。
- 成長分化因子:Growth-differentiation factor 3 (Gdf3) は、Nodalシグナル経路に関与し、AVEの形成に重要な役割を果たします。
- その他の分泌因子:Bone morphogenetic protein 4 (Bmp4) は、AVEの発達と移動を調節するシグナル分子です。
マウスの初期胚において、AVEはWntシグナルを拮抗するSfrp5やDkk1、TGFβシグナルを抑制する因子、そしてGdf3などの成長分化因子を分泌します。これらの分子は、胚の前後軸の形成やAVEの発達に重要な役割を果たします。(consensus.ai)
マウスの原始線条
マウスの胚はカップ状になっているため、原始線条のイメージが湧きにくく、他との比較もしにくいと思いますが、下の論文の図ではカップを平らにした状態のイメージ図も描かれており、理解を助けてくれます。
Axis Development and Early Asymmetry in Mammals Cell Volume 96, Issue 2p195-209 January 22, 1999 https://www.cell.com/fulltext/S0092-8674%2800%2980560-7
マウス胚での中胚葉誘導の始まり:extraembryonic ectodermからのBMP4シグナル
- At the blastocyst stage, the polar TE lies adjacent to the epiblast and is fated to form the extraembryonic ectoderm and ectoplacental cone, which will subsequently form the fetal portion of the placenta, whereas the mural TE initially encloses the blastocyst cavity and eventually forms the outer layer of the parietal yolk sac.
- Between E5.5 and E6.0, the proamniotic cavity expands to the extraembryonic ectoderm, forming the proamniotic canal.
- https://www.sciencedirect.com/topics/engineering/extraembryonic-ectoderm
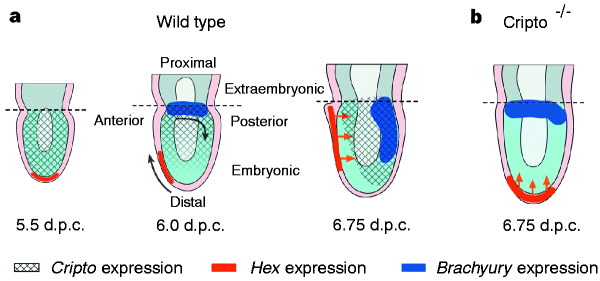
下の図のシグナル分子のまとめが非常にわかりやすいと思いました。しかし緑の背景に白抜きの文字は読みにくい。extraembryonic ectodermがBMP4を分泌し、BMP受容体1がそのシグナルを受容して、結果として中胚葉マーカーであるT遺伝子が発現します。これは尾部で起こります。ところが、吻側においては、HEX遺伝子を発現するDistal Visceral Endoderm (DVE)が前方へと移動してAnterior Visceral Endoderm (AVE)になっているのですが、AVEからはBMPシグナルを阻害する因子が分泌されており、中胚葉誘導が阻害されるのです。
Axis Development and Early Asymmetry in Mammals Cell Volume 96, Issue 2p195-209January 22, 1999 https://www.cell.com/fulltext/S0092-8674%2800%2980560-7
- The role of BMP4 signaling in trophoblast emergence from pluripotency Cellular and Molecular Life Sciences 25 July 2022 Volume 79, article number 447, (2022)
マウス Distal Visceral Endoderm (DVE)におけるHEX遺伝子の発現
原腸形成に至る流れの始めの段階で生じる変化の一つは、DVEでのHEX遺伝子の発現です。
- Jeremy Davis. Life Unfolding
下の写真のB,C,D,EではHEX遺伝子の原始内胚葉 primitive endodermでの発現が示されています。B,Cの時期はまだカップ状になっていませんが、Dの時期にはカップ状の極の部分に位置しています。さらにE,F,Gとステージが進むと前側にHEX発現細胞が移動しているのがよくわかります。Gの時期には中胚葉マーカーであるT遺伝子が尾側に発現しています。
Fig.1 Whole-mount in situ hybridisation analysis showing asymmetrical Hex expression in the visceral endoderm of pregastrulation stage embryos. (A-C) 4.5 dpc blastocysts showing Oct-4 expression (A) in the inner cell mass and Hex expression (B,C) in the primitive endoderm (black arrowhead). (D,E) Hex expression (black arrowhead) in the distal tip visceral endoderm of 5.5 dpc embryos. Note that the Hex expression domain is immediately proximal to the distal tip in the slightly older embryo shown in E. (F,G) Double in situ hybridisation analysis of Hex (black arrowhead) and T (white arrowhead) at 6.0 dpc (F) and 6.5 dpc (G). Hex expression at 6.0 dpc is clearly asymmetrical within the endoderm prior to the accumulation of T transcripts to the nascent primitive streak at the posterior pole of the embryo (G). Bar, 40 μm (A-C); 50 μm (D); 60 μm (E-G).
Hex: a homeobox gene revealing peri-implantation asymmetry in the mouse embryo and an early transient marker of endothelial cell precursors Development (1998) 125 (1): 85–94. 01 January 1998
BMP4シグナルは生殖細胞の形成にも必要だそうです。
- 生殖細胞の起源 http://ikagaku.jp/wp-admin/post.php?post=12454&action=edit
ヒトにextraembryonic ectodermはあるか?
In humans, a structure equivalent to the mouse extraembryonic ectoderm is not thought to form.
Pluripotent Stem Cells Susana M. Chuva de Sousa Lopes, Christine L. Mummery, in Handbook of Stem Cells (Second Edition) , 2013 https://www.sciencedirect.com/topics/engineering/extraembryonic-ectoderm
In the post-implantation mouse embryo, formation of the PS and initiation of germ layer formation are driven by signaling activities emanating from the extraembryonic tissues such as the extraembryonic ectoderm, which gives rise to chorionic ectoderm, and visceral endoderm and from within the epiblast. However, in primates, there is no clear equivalent to the TE-derived extraembryonic ectoderm of the mouse, which is a major source of BMP to induce gastrulation and primitive-streak formation. In humans and nonhuman primates, the amnion (amniotic epithelium) is reputed to behave like a signaling center of BMP activity to induce the differentiation of mesoderm in the Cynomolgus embryonic disc and the human PASE (Shao et al., 2017; Yang et al., 2021; Zheng et al., 2019). https://www.cell.com/developmental-cell/pdf/S1534-5807(21)01042-X.pdf
そうなるとヒトの場合は、どこから最初のシグナルが来るの?という疑問が湧きます。
Early mouse embryonic development. As described in the text, the blastocyst at 3.5 dpc consists of two tissues: the inner cell mass and trophectoderm. At 4.5 dpc the primitive endoderm is formed and the embryo starts to implant into the uterine wall. By 5.5 dpc, the embryo is cup-shaped https://www.researchgate.net/figure/Early-mouse-embryonic-development-As-described-in-the-text-the-blastocyst-at-35-dpc_fig1_228357767
BMP4シグナル
Front Cell Dev Biol. 2024 Apr 22;12:1386739. doi: 10.3389/fcell.2024.1386739 A comprehensive review: synergizing stem cell and embryonic development knowledge in mouse and human integrated stem cell-based embryo models https://pmc.ncbi.nlm.nih.gov/articles/PMC11074781/
上皮ー間葉転換 EMT
- Basal delamination during mouse gastrulation primes pluripotent cells for differentiation
 https://pmc.ncbi.nlm.nih.gov/articles/PMC7616279/
https://pmc.ncbi.nlm.nih.gov/articles/PMC7616279/
細胞移動に必要な分子
人間が歩くときに、片足で地面をつかんで、もう一方の足を前にやり、こんどはその足で地面をつかんで、もう一方の足を前に出すということを繰り返します。細胞も全く同じで、足場に接着し、全体を動かし、足場から離れて、また次の足場にくっつきます。分子としてh、Cdc42, Rac, Rhoなどが関与するよです(下の動画参照)。
Cell locomotion | cell motility | cell migration | Rho-Rac-Cdc42 signaling in cell locomotion Animated biology With arpan チャンネル登録者数 27.8万人
Cdc42は細胞の極性を維持し、細胞が動く方向を制御しているそうです。chemoatractantを与えて細胞を引き寄せる実験において、Cdc42が働かないようにした細胞は、細胞の移動はできるが、方向性を失うのだそう。
Racはアクチンの重合を促進して細胞の足を前に突き出して、インテグリンと細胞外基質との接着も行う。
Developmental Biology Volume 265, Issue 1, 1 January 2004, Pages 23-32 Developmental Biology Review Cell migration: Rho GTPases lead the way https://www.sciencedirect.com/science/article/pii/S001216060300544X
下の図が、細胞が移動するときの各ステップを分解して説明しているので分かりやすいです。
S
Signaling networks of Rho GTPases in cell motility May 2013Cellular Signalling 25(10) DOI: 10.1016/j.cellsig.2013.04.009 SourcePubMed LicenseCC BY-NC-ND 3.0 Samer J HannaSamer J HannaMirvat El-Sibai https://www.researchgate.net/publication/236739227_Signaling_networks_of_Rho_GTPases_in_cell_motility
細胞移動におけるCdc42, Rac, Rhoの役割
Cdcc42, Rac, RhoはどれもRho低分子GTP結合タンパク質のファミリーメンバーです。いずれも細胞骨格の制御に関与します。
Distinct predictive performance of Rac1 and Cdc42 in cell migration ScienceVio チャンネル登録者数 8620人 Published: 04 December 2015 Distinct predictive performance of Rac1 and Cdc42 in cell migration Masataka Yamao, Honda Naoki, Katsuyuki Kunida, Kazuhiro Aoki, Michiyuki Matsuda & Shin Ishii Scientific Reports volume 5, Article number: 17527 (2015)
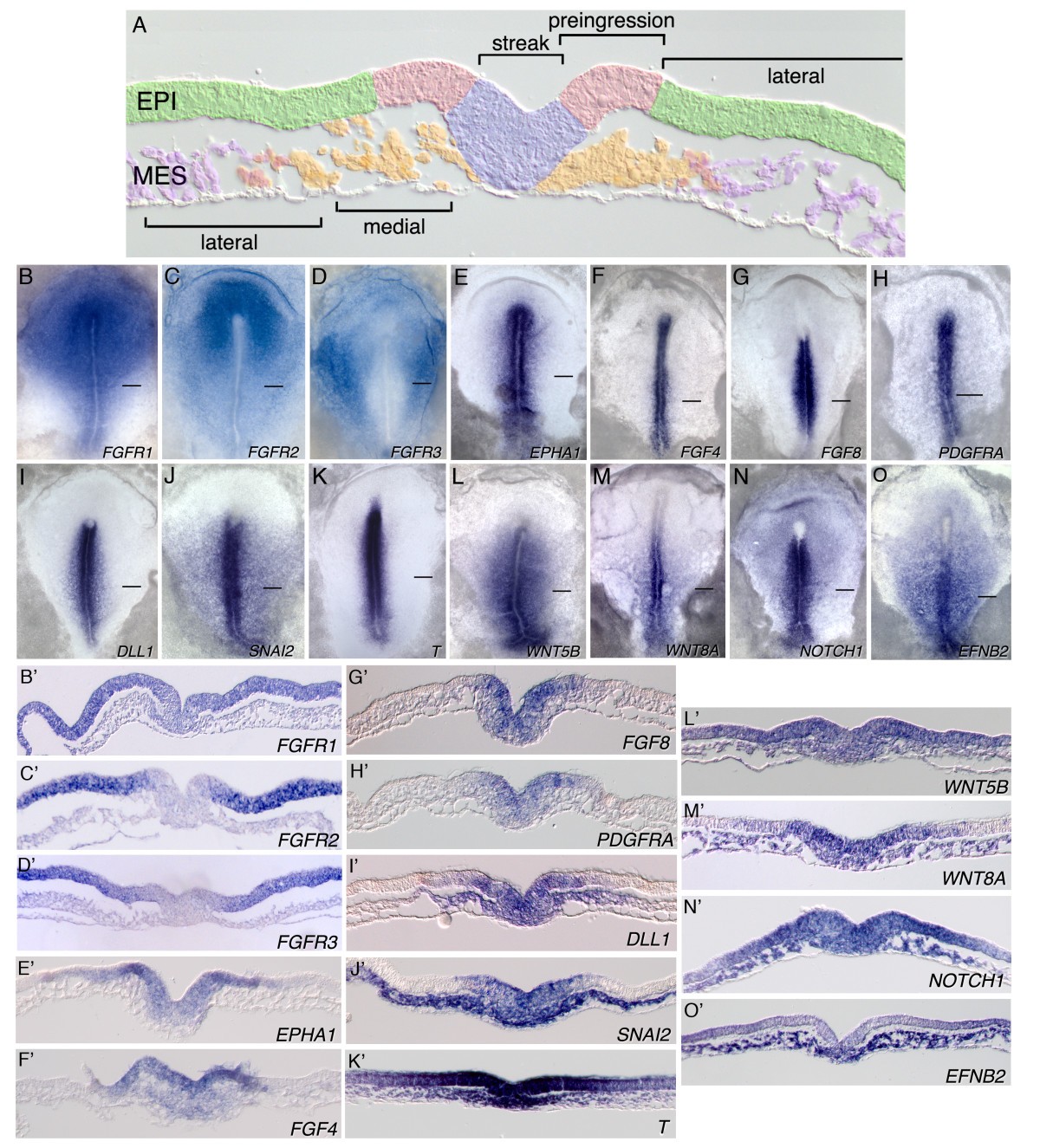
ニワトリ胚の中胚葉細胞の移動経路
- Cell movement patterns during gastrulation in the chick are controlled by positive and negative chemotaxis mediated by FGF4 and FGF8 Dev Cell . 2002 Sep;3(3):425-37. doi: 10.1016/s1534-5807(02)00256-3. https://www.cell.com/developmental-cell/fulltext/S1534-5807(02)00256-3 蛍光標識した 原始結節(node)の移植片の細胞の移動経路を経時的に観察
- Fates and migratory routes of primitive streak cells in the chick embryo Delphine Psychoyos, Claudio D. Stern Author and article information Development (1996) 122 (5): 1523–1534. 01 May 1996 https://journals.biologists.com/dev/article/122/5/1523/39034/Fates-and-migratory-routes-of-primitive-streak
ニワトリ胚のFGFの役割
FGF signalling through RAS/MAPK and PI3K pathways regulates cell movement and gene expression in the chicken primitive streak without affecting E-cadherin expression Katharine M Hardy, Tatiana A Yatskievych, JH Konieczka, Alexander S Bobbs & Parker B Antin BMC Developmental Biology volume 11, Article number: 20 (2011) Published: 21 March 2011 https://bmcdevbiol.biomedcentral.com/articles/10.1186/1471-213X-11-20
Gene expression pattern Expression of Fgf4 during early development of the chick embryo Mechanisms of Development Volume 85, Issues 1–2, 1 July 1999, Pages 189-192 https://www.sciencedirect.com/science/article/pii/S0925477399000933
- FGF Signaling Regulates Mesoderm Cell Fate Specification and Morphogenetic Movement at the Primitive Streak Developmental Cell Volume 1, Issue 1p37-49July 2001 https://www.cell.com/developmental-cell/fulltext/S1534-5807(01)00017-X
マウス胚のFGFの役割
- Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo Genes & Dev. 1999. 13: 1834-1846 https://genesdev.cshlp.org/content/13/14/1834/F1.expansion.html
AK Hadjantonakis: Cell lineage specification & tissue morphogenesis in the early mouse embryo. Genetics Society of America チャンネル登録者数 2040人
CerberusやDickkopfによるWntシグナリングの阻害
Heads or tails: Wnts and anterior–posterior patterning Terry P Yamaguchi Current Biology Volume 11, Issue 17pR713-R724 September 04, 2001 https://www.cell.com/current-biology/fulltext/S0960-9822%2801%2900417-1
nordal
The ability of the Nodal pathway to induce both mesoderm specification and axis extension in explants is consistent with its role in vivo, where Nodal is necessary for both (37–40). For example, mouse embryos mutant for Nodal signaling components fail to gastrulate entirely (41). Zebrafish embryos lacking all Nodal function – through loss of the coreceptor Tdgf1/Cripto (MZoep-/-) (40), ligands (sqt-/-cyc-/-) (38), or downstream effector Smad2 (MZsmad2-/-) (42) – similarly lack all endoderm and most mesoderm and undergo abnormal gastrulation movements resulting in a severely shortened AP axis.
Temporal dynamics of BMP/Nodal ratio drive tissue-specific gastrulation morphogenesis https://www.biorxiv.org/content/10.1101/2024.02.06.579243v1.full
Eomes, Criptなどのシグナル分子
From fertilization to gastrulation: axis formation in the mouse embryo. Curr Opin Genet Dev . 2001 Aug;11(4):384-92. DOI:10.1016/S0959-437X(00)00208-2Corpus ID: 17925624
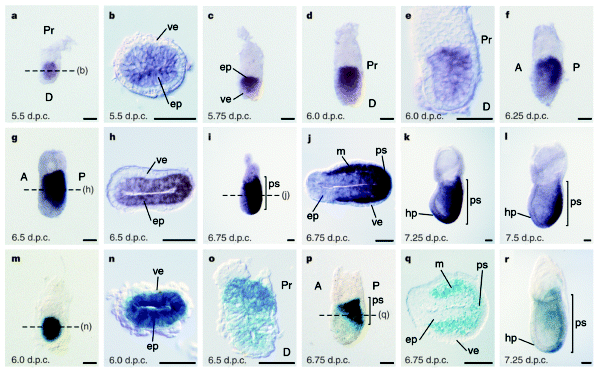
a–l, Whole-mount in situ hybridization analysis. a, Uniform symmetric staining in the epiblast at 5.5 d.p.c.; b, a cross section shows lack of expression in the visceral endoderm. c, d, Proximal–distal gradient of expression in the epiblast. e, Sagittal section of d. f, g, Expression shifts caudally before the onset of gastrulation at 6.5 d.p.c. h, Cross-section of g shows widespread expression in the epiblast and no expression in the visceral endoderm. i, Mid-streak stage; j, cross-section shows intense staining in the newly formed embryonic mesoderm. k, l, Expression persists in the primitive streak and head process at the neural plate stage. m–r, β-Galactosidase staining of Cripto heterozygotes. m, n, Uniform staining in the epiblast before gastrulation. o, Sagittal section shows proximal–distal graded staining just before gastrulation. p, Early-streak stage embryo, and q, cross-section. r, Early neural-plate-stage embryo: note more intense staining at distal end of the primitive streak. In all panels, anterior faces to the left when anterior–posterior orientation can be identified; staging before primitive streak formation is approximate. Scale bars, 0.05 mm. A, anterior; D, distal; ep, epiblast; hp, head process; m, mesoderm; P, posterior; Pr, proximal; ps, primitive streak (bar denotes extent of streak); ve, visceral extra-embryonic endoderm.
Cripto is required for correct orientation of the anterior–posterior axis in the mouse embryo Nature volume 395, pages702–707 (1998) Published: 15 October 1998 https://www.nature.com/articles/27215
- The Dynamics of Morphogenesis in the Early Mouse Embryo. Cold Spring Harbor Perspectives in Biology, 26 Jun 2014, 7(11):a015867 https://doi.org/10.1101/cshperspect.a015867 PMID: 24968703 PMCID: PMC4277506

- Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse J. Rossant, P. Tam Published in Development 1 March 2009 Biology DOI:10.1242/dev.017178Corpus ID: 207151163

Vg1
- Molecular mechanisms controlling the biogenesis of the TGF-β signal Vg1 PNAS October 16, 2023 120 (43) e2307203120 https://www.pnas.org/doi/10.1073/pnas.2307203120 The TGF-beta signals Nodal and Vg1 (Dvr1/Gdf3) play crucial roles in vertebrate development (1, 2), including the induction of mesendoderm and the generation of left-right asymmetry (3–17). For example, secreted Vg1-Nodal heterodimers induce a gradient of signaling that patterns the embryonic mesendoderm in zebrafish (10). Vg1-Nodal heterodimers exert their effects as ligands for a receptor complex that comprises Activin serine-threonine kinase receptors and an essential coreceptor called Oep (Tdgf1/CRIPTO) (18–20). Activated ligand-receptor complexes catalyze phosphorylation of Smad2 (pSmad2), which accumulates in the nucleus to induce the expression of mesendodermal genes (21).
中胚葉マーカー分子
Brachyury (TBXT (human), also T/Bra (mouse))
- TBXT dose sensitivity and the decoupling of nascent mesoderm specification from EMT progression in 2D human gastruloids bioRxiv [Preprint]. 2023 Nov 9:2023.11.06.565933. [Version 2] doi: 10.1101/2023.11.06.565933
- The T gene is necessary for normal mesodermal morphogenetic cell movements during gastrulation Development (1995) 121 (3): 877–886. the phenotype of homozygous mutant mouse embryos does not obviously correlate with an essential function for T during the early stages of gastrulation, since development rostral to the forelimb bud appears grossly normal. Only in more caudal trunk regions and in later embryos is the notochord missing and other mesodermal derivatives deficient or defective (Herrmann, 1992; Beddington et al., 1992; Rashbass et al., 1994). https://journals.biologists.com/dev/article/121/3/877/38504/The-T-gene-is-necessary-for-normal-mesodermal 機能的に重要そうなのにノックアウトマウスの表現型は非常にささやか。
- A cell autonomous function of Brachyury in T/T embryonic stem cell chimaeras Nature volume 353, pages348–351 (1991) https://www.nature.com/articles/353348a0
- Expression pattern of the mouse T gene and its role in mesoderm formation. Nature, 343(6259), 657–659. 10.1038/343657a0 Wilkinson D. G., Bhatt S., & Herrmann B. G. (1990). https://www.nature.com/articles/343657a0 有料
- Effects of the brachyury (T) mutation on morphogenetic movement in the mouse embryo Dev Biol . 1981 Oct 30;87(2):242-8. doi: 10.1016/0012-1606(81)90147-0. 有料



80560-7/asset/c406199d-b96b-4fea-9e41-d280ff70ea70/main.assets/gr1.jpg)
80560-7/asset/baa0b33b-7950-4e15-b02c-b80aaa387e9e/main.assets/gr2.jpg)





00417-1/asset/81432966-fc4a-450c-92e2-116d185449b6/main.assets/gr2.jpg)
00417-1/asset/dcd307b3-40eb-4e07-b401-f15cb71aed06/main.assets/gr5.jpg)